Producing sufficient ammonia to sustain global food production carries a significant carbon footprint; a new UB-led study outlines a process that could help address this issue.
Producing sufficient ammonia to sustain global food production carries a significant carbon footprint; a new UB-led study outlines a process that could help address this issue.

This groundbreaking industrial reaction, which combines hydrogen and nitrogen to create ammonia, is the foundation of synthetic fertilizers that feed much of the global population. It played a critical role in enabling the population boom of the past century.
However, this process also poses a significant threat to future generations. It accounts for about 2% of the world’s energy consumption, and the hydrogen it requires is largely derived from fossil fuels.
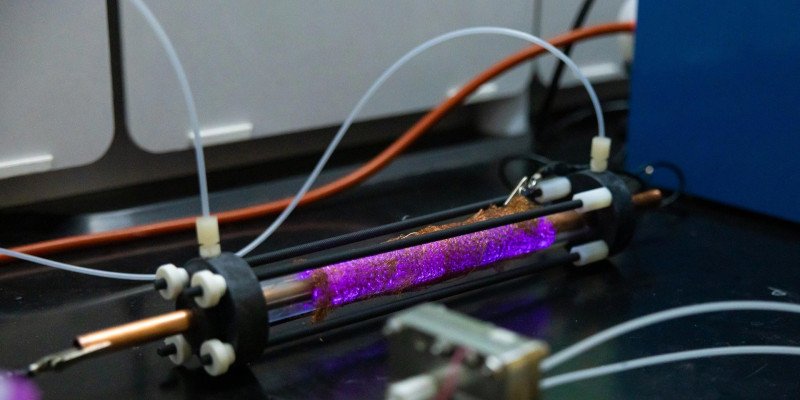
Inspired by nature’s own methods of producing ammonia—such as through lightning—a research team led by the University at Buffalo has developed a revolutionary reactor. This device produces ammonia directly from nitrogen in the air and water with zero carbon emissions.
The plasma-electrochemical reactor, detailed in a study published in the Journal of the American Chemical Society, operates at room temperature and achieves a sustained ammonia production rate of about 1 gram per day for over 1,000 hours—all without reliance on fossil fuels.
The researchers say this is a significant advance toward green ammonia synthesis at an industrially competitive production rate and reaction stability.
“Ammonia is often considered the chemical that feeds the world, but we also have to face the realization that the Haber-Bosch process has not been modernized since its invention 100 years ago. It still uses a high-temperature, high-pressure processing, and generates a large carbon footprint, making it unsustainable for the long term,” says the study’s corresponding author, Chris Li, PhD, assistant professor of chemistry in the UB College of Arts and Sciences. “Our process only requires air and water, and can be powered by renewable electricity.”
Mimicking nature’s nitrogen cycle
Nature has its own way of producing fertilizer.
In nitrogen fixation, the electrical energy of a lightning strike breaks up nitrogen molecules in the atmosphere to form different nitrogen oxide species. After falling down as rainwater, nitrogen oxides are converted into ammonia by bacteria in the soil, supplying plants with nutrients.
In the UB-led team’s two-step reactor, the role of lightning is replaced with plasma, and the role of bacteria is replaced by a catalyst of copper-palladium.
“Our plasma reactor converts humidified air into nitrogen oxide fragments, which are then placed in an electrochemical reactor that uses the copper-palladium catalyst to convert them into ammonia,” Li says.
Crucially, the catalyst is able to adsorb and stabilize the numerous nitrogen dioxide intermediates created by the plasma reactor. The team’s graph theory algorithm identified that most the nitrogen oxide compounds have to cycle through nitric oxide or amine as an intermediate step before becoming ammonia. This allowed the team to intelligently design a catalyst that binds favorably with those two compounds.
“When plasma energy or a lightning strike activates nitrogen, you generate a soup of nitrogen oxide compounds. To simultaneously convert, in our case, up to eight different chemical compounds into ammonia is incredibly difficult,” says Xiaoli Ge, the study’s first author and a postdoctoral researcher in Li’s lab. “Graph theory essentially allows us to map out all the different reaction paths and then identify a bottleneck chemical. We then optimize our electrochemical reactor to stabilize the bottleneck chemical, so that all the different intermediates will be selectively conferred into ammonia.”
Scaling up
Li’s team is currently in the process of scaling up their reactor and is exploring both a startup and partnerships with industry to help commercialize it. UB’s Technology Transfer Office has filed a patent application on the reactor and methods for its use.
Over half the world’s ammonia is produced by four countries — China, the United States, Russia, and India — while many developing countries are unable to produce their own. While the Haber-Bosch process must be conducted on a large scale in a centralized power plant, Li says their system can be done at a much smaller scale.
“You can imagine our reactors in something like a medium-sized shipping container with solar panels on the roof. This can then be placed anywhere in the world and generate ammonia on demand for that region,” he says. “That’s a very exciting advantage of our system, and it will allow us to produce ammonia for underdeveloped regions with limited access to the Haber-Bosch process.”
Reference: “Controlling the Reaction Pathways of Mixed NOxHy Reactants in Plasma-Electrochemical Ammonia Synthesis” by Xiaoli Ge, Chengyi Zhang, Mayuresh Janpandit, Shwetha Prakash, Pratahdeep Gogoi, Daoyang Zhang, Timothy R. Cook, Geoffrey I.N. Waterhouse, Longwei Yin, Ziyun Wang and Yuguang C. Li, 12 December 2024, Journal of the American Chemical Society.
Type and Press enter