There are times when it just isn’t efficient enough to send samples to the lab for testing. Richard Crocombe outlines advances in portable spectroscopy and which instruments to use for rapid, application-specific onsite analysis.
There are times when it just isn’t efficient enough to send samples to the lab for testing. Richard Crocombe outlines advances in portable spectroscopy and which instruments to use for rapid, application-specific onsite analysis.

Over the past 20 years, advances in consumer electronics (e.g., all the components in smartphones), micromachining (MEMS), and optical telecommunications have come together to enable spectrometers to be made portable. These instruments now range in size from that of a cordless drill to smaller than a deck of playing cards. These small and ruggedized spectrometers are now routinely used outside the traditional laboratory to give answers in the field – at the point of need. This has transformed operations within companies, and in some cases, entire businesses.
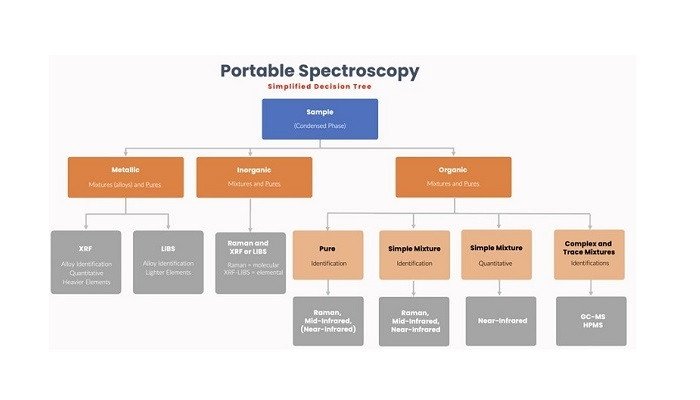
The major portable spectroscopy techniques can be divided into three classes: elemental analysis (x-ray fluorescence and laser-induced breakdown spectroscopy); optical molecular analysis (UV-visible, near-infrared, mid-infrared, and Raman); and mass spectrometry (high-pressure mass spectrometry and gas chromatography-mass spectrometry). Each of these classes has its own specific abilities and applications, and it’s obviously important to choose the right technique for the problem at hand.
Portable X-ray fluorescence (XRF) is a well-established technique and has been used for at least twenty years throughout the whole of the metals business life cycle: from mineral prospecting, mining, refining, raw material confirmation, in-process inspection, and finally to scrap metal sorting.
Other applications include the detection of heavy metals (e.g., lead) in old house paint and imported toys, the ‘cash for gold’ business, environmental surveys, and cultural heritage studies. Portable XRF has some limitations for elements lighter than silicon and the newer portable technique of laser-induced breakdown spectroscopy (LIBS) is especially useful in these instances, for tasks such as aluminum alloy sorting and lithium applications.
Rapid identification of chemical substances, often ‘white powders’, is critical in a number of different scenarios, from incoming material confirmation in pharmaceutical manufacturing, detection of counterfeit pharmaceuticals in the field, to the identification of suspicious materials by the police, customers, and border officials, and airport security personnel. The value of instant identification is strikingly apparent for a hazardous material spill.
Here, a technician wearing protective gear has to rapidly characterize spilled material – is it flammable, poisonous, corrosive, volatile, explosive, etc.? This cannot be achieved by sending a sample to a lab; it has to be done on the spot with a reliable result, and confidence in the actions required to remediate the spill.
Near-infrared (NIR), mid-infrared, and Raman spectrometers are all used in these areas, but each has its strengths and weaknesses. Briefly, Raman is often preferred because of its ‘point-and-shoot’ ability, whereas mid-infrared typically requires the material to be handled. This tends to outweigh the issue of fluorescence in Raman spectra, and instrument manufacturers have implemented a variety of schemes to mitigate these effects.
Both techniques yield very specific spectroscopic information. NIR spectroscopy has been used for many years for the quantitative analysis of natural products (grain, fruits, etc.), and it is well-suited to highly heterogeneous materials.
It should be noted that the optical techniques are limited to major component (per cent-level) analyses in condensed phases. For trace and complex mixture analyses, mass spectrometric (MS) techniques are required. MS has typically required the use of a high vacuum, and this precluded miniaturization of the instrument to a handheld format.
However, one variety of mass spectrometry – ion mobility spectrometry (IMS) has been used in airport security applications since the late 1980s. If your luggage was ‘swabbed’, and the swab taken over to a small desktop instrument, then that was an IMS. These instruments are also available as handhelds, typically resembling a ‘dustbuster-type’ portable vacuum cleaner.
Briefcase-sized gas chromatography-mass spectrometers (GC-MS) have been commercially available since about 1996. They are widely used by hazardous material technicians, the military, and environmental scientists to investigate and characterize suspicious areas.
These instruments typically employ narrow-bore GC columns and small ion trap or quadrupole spectrometers, and these, in turn, allow the use of miniature pumps that permit portable operation even where the MS itself demands high vacuum operation. Because these instruments use a separations front end, and mass spectrometric detection, they are excellent for analyzing complex mixtures, and for detecting trace components within those samples.
A recent entrant is high-pressure mass spectrometry (HPMS), using an array of micro-ion-traps, which require only a modest vacuum (0.1 atmospheres), permitting a smaller pump and a handheld format. HPMS is fast (seconds), reliable for programmed target chemicals, and provides a presumptive method for identification.
The commercially available instrument has “Hunter” modes, which optimize the analytical settings for specific types of targets, e.g., drug-hunter mode, chemical-warfare-agent-hunter mode, and explosives-hunter mode.

The current generation of commercial portable spectrometers is smaller, lighter, but more powerful than their predecessors of just five years ago, and their abilities for both qualitative and quantitative analyses have increased significantly.
This field has seen rapid development in a self-perpetuating circular fashion: the desire to perform analyses in the field has driven the development of portable instruments, while their availability has driven the development of new applications. Therefore, more and more applications will move from the lab to the loading dock and out into the field, giving actionable answers at the point of need.
Portable optical spectrometers continue to get smaller, some the size of a fingertip, with availability at a lower cost. In addition, a variety of multi-spectral sensors are available, with, for instance, sixteen separate spectral band passes in the visible and near-infrared.
Molecular absorption bands in those regions are often very broad so these sensors can be used for some material identification applications. Intriguingly, the reductions in size and cost now permit miniature spectrometers, spectral sensors, and other photonic components to be embedded in some consumer products.
In the next few years, we will see the growth of ‘smart’ products containing these optical devices in white goods (refrigerators, washers, dryers, ovens, vacuum cleaners), personal care (toothbrushes, hair care, cosmetics), and fitness (sports watches, smart watches) sectors.
Finally, it’s worth noting that the ultimate transportable (if not person-portable) spectrometers are in the latest generation of Mars rovers, which combine both Raman and LIBS spectrometers.
Type and Press enter